Biotechnology in Victoria: the Public Sector's Investment
Overview
In its role as the public sector agency responsible for administering the bulk of public investment in biotechnology, the Department of Business and Innovation (DBI) has delivered a range of world class facilities and technology platforms for the biotechnology and broader life sciences sector.
DBI has publicly reported that, of the 16 high-level strategic goals and targets that the government set for the biotechnology sector over the last decade, ten have been met, and adequate progress has been made for targets that have not been fully met.
Notwithstanding the claimed achievement of high-level sector goals and targets, there are shortcomings in DBI’s approach to investment targeting, and gaps in its benefits measurement and monitoring approaches.
DBI’s administrative focus for the sector is also mainly on grant and contract compliance. Though useful in its own right, this focus does not produce performance data about whether the strategic aims of the public sector’s investment in the biotechnology sector are being delivered.
As a result, after more than ten years of active investment and ‘market intervention’ in the biotechnology sector, DBI is not yet able to objectively demonstrate the development of a cause and effect relationship between its investments in the biotechnology sector and the results achieved in that sector.
Biotechnology in Victoria: the Public Sector's Investment: Message
Ordered to be published
VICTORIAN GOVERNMENT PRINTER August 2011
PP No 53, Session 2010–11
President
Legislative Council
Parliament House
Melbourne
Speaker
Legislative Assembly
Parliament House
Melbourne
Dear Presiding Officers
Under the provisions of section 16AB of the Audit Act 1994, I transmit my performance report on Biotechnology in Victoria: the Public Sector's Investment.
Yours faithfully
![]()
D D R PEARSON
Auditor-General
17 August 2011
Audit summary
Biotechnology is a collection of technologies, techniques, and tools that use living organisms and their components to make new products and to develop new industrial processes. More broadly, biotechnology is a 'life science'-the study of the structure, behaviour and life processes of living organisms such as plants, animals and human beings.
Since 1999 to the end of the 2011 financial year, Victoria has invested $3.442 billion of public funds in the science, technology and innovation (STI) sector. Over the last decade the biotechnology sector, which is a part of the STI sector, has directly received some $722 million in public sector investment, or 21 per cent of total innovation funding.
In 2001 the former Department of Innovation, Industry and Regional Development, now known as the Department of Business and Innovation (DBI), developed the first whole-of-government Biotechnology Strategic Development Plan for Victoria (BSDP). Two more plans followed in 2004 and 2007.
Many investments in the biotechnology sector are intrinsically worthwhile, because they are aimed at extending and improving human life. This does not, however, exempt these public investments from scrutiny of their rationale, efficacy, benefits or outcomes.
Although precise measurement can be challenging, due to the interaction of biotechnology with the innovation economy, it is reasonable to expect that:
- the potential benefit from each public investment is identified
- the methods chosen to achieve the benefit are relevant, appropriate and reliable
- costs incurred are economical and represent value for money in the context
- the outcomes of the investment are progressively measured and assessed so that achievements can be attributed, and lessons learned to inform future investment decisions.
The objective of this audit was to examine the efficiency and effectiveness of the Victorian public sector's investments in the biotechnology sector.
There were three sub-objectives to the audit, which were addressed by posing the following questions:
- Is there clear alignment of programs and investments with the applicable BSDP?
- Are program and investment outputs monitored, analysed, and reported to assess the achievement of desired strategic outcomes?
- Have available benefits been realised?
The audit examined the application of the BSDPs as well as program design, program governance, investment evaluations, business case reviews, progress reporting, and benefits assessments. The audit also examined other documentation and data relating to DBI's biotechnology activities, as well as advice provided to the government by the Department of Treasury and Finance and the Department of Premier and Cabinet.
The following public sector agencies were included in this audit:
- Australian Synchrotron Company Limited
- Australian Synchrotron Holding Company Limited
- Bio21 Molecular Science and Biotechnology Institute (and its parent institution, the University of Melbourne)
- DBI
- Melbourne Health (as responsible entity for the BioGrid project).
Conclusions
In its role as the public sector agency responsible for administering the bulk of public investment in biotechnology, DBI has delivered a range of world class facilities and technology platforms for the biotechnology and broader life sciences sector.
The eight biotechnology projects examined have directly supported life sciences researchers and practitioners and have the potential to:
- stimulate scientific research and innovation
- assist pharmaceutical and medical discoveries
- attract more private investment
- develop highly skilled people
- extend existing scientific knowledge.
DBI has publicly reported that of the 16 high-level strategic goals and targets that the government set for the biotechnology sector over the last decade, ten have been met, and adequate progress has been made for targets that have not been fully met.
Notwithstanding the claimed achievement of high-level sector goals and targets, there are shortcomings in DBI's approach to investment targeting, and gaps in its benefits measurement and monitoring approaches. In particular, DBI does not use recognised or accepted methodologies to systematically determine portfolio risk and inform investment allocations.
DBI's administrative focus for the sector is also mainly on grant and contract compliance. Though useful in its own right, this focus does not produce performance data about whether the strategic aims of the public sector's investment in the biotechnology sector are being delivered. Instead, the primary focus of DBI's performance measurement is monitoring the impact of short-term stimulatory activity in the sector, without evident attention to the delivery of longer-term and strategic benefits.
As a result, after more than ten years of active investment and 'market intervention' in the biotechnology sector, DBI is not yet able to objectively demonstrate the development of a cause and effect relationship between its investments in the biotechnology sector and the results achieved in that sector. It is, however, reasonable to attribute at least part of the achievement of BSDP targets to investments made by the public sector.
Against a background of typically long lead times for the full benefits from such investments to become evident, it is now timely for DBI to address these issues so that progressive assurance can be provided that its portfolio of investments in biotechnology is both targeted to where it is needed and is achieving its intended purpose.
Further, because there are no measures or targets that could be used as a benchmark against other leading international biotechnology locations, it is not possible to conclude on the aspirational goal that Victoria is, or will be, 'one of the world's top five biotechnology locations'.
Findings
Based on our review of eight representative investments in the biotechnology sector, there are consistent systemic issues in relation to measuring benefits. These issues can be summarised as follows:
- monitoring and evaluation of benefits is limited
- benefits from investments are not always sustainable
- DBI can not systematically demonstrate whether benefits have been realised.
Monitoring and evaluation of benefits
Because DBI's benefits measurement and management framework is not comprehensive or robust, DBI is not able to objectively demonstrate whether benefits have been achieved from public sector investments in biotechnology.
Sustainability of realised benefits
Notwithstanding the achievements recorded, DBI's preferred funding models do not explicitly require the identification and pursuit of sustainable future benefits.
Achievement of benefits
In terms of benefits measurement, we observed:
- reporting is at a very high level and often based on grantee data that has not been validated
- a baseline of a current or 'before' state is not set so that the 'after' state can be accurately measured
- data collected by DBI has been focused on sector-level metrics that do not measure the efficacy of individual grants
- retrospective studies commissioned by DBI have methodological weaknesses in that they use general economic 'activity' as a proxy for specific public sector investment 'effectiveness'.
In consequence, reliable conclusions cannot be drawn regarding the extent to which intended objectives are being met in a timely and cost-effective manner.
Recommendations
-
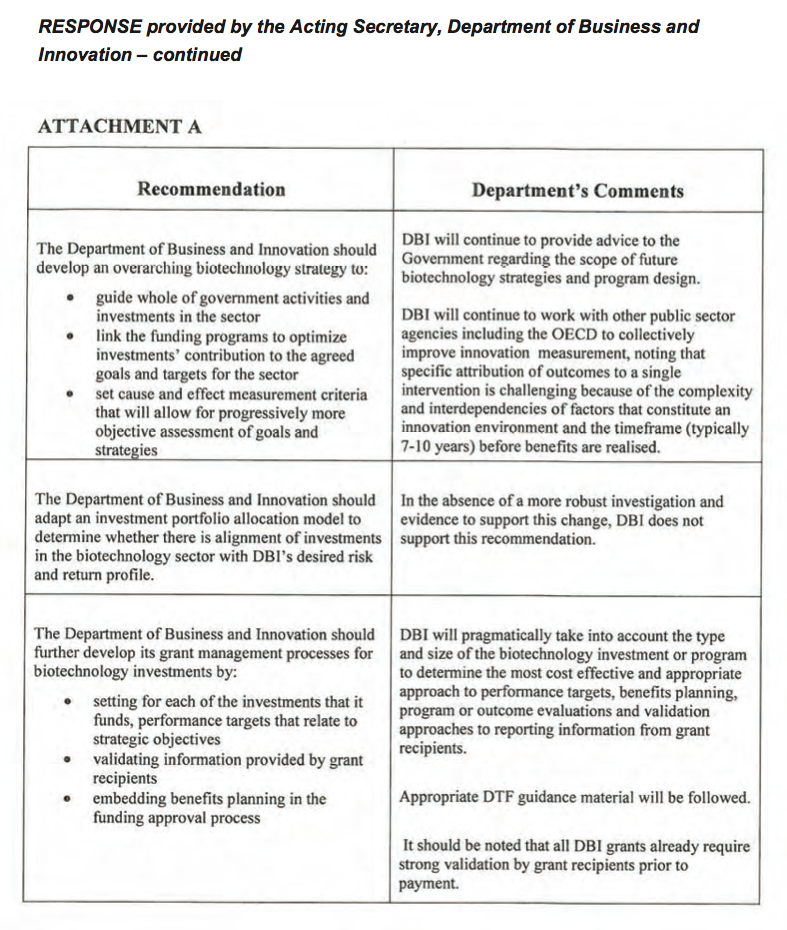
The Department of Business and Innovation should develop an overarching biotechnology strategy to:
- guide whole-of-government activities and investments in the sector
- link the various funding programs to optimise investments' contribution to the agreed goals and targets for the sector
- set cause and effect measurement criteria that will allow for progressively more objective assessment of the achievement of goals and strategies.
-
The Department of Business and Innovation should adapt an investment portfolio allocation model to determine whether there is alignment of investments in the biotechnology sector with the Department of Business and Innovation's desired risk and return profile.
-
The Department of Business and Innovation should further develop its grant management processes for biotechnology investments by:
- setting, for each of the investments that it funds, performance targets that relate to strategic objectives
- validating information provided by grant recipients
- embedding benefits planning in the funding approval process.
Submissions and comments received
In addition to progressive engagement during the course of the audit, in accordance with section 16(3) of the Audit Act 1994 a copy of this report was provided to the Australian Synchrotron Company Limited, Australian Synchrotron Holding Company Limited, Department of Business and Innovation, Melbourne Health, and the University of Melbourne with a request for submissions or comments.
Agency views have been considered in reaching our audit conclusions and are represented to the extent relevant and warranted in preparing this report. Their full section 16(3) submissions and comments are included in Appendix C.
1 Biotechnology and the Victorian public sector
1.1 Introduction
Biotechnology comprises the technologies, techniques and tools that use living organisms and their elements to make products and develop new industrial processes. It is a 'life science'-the study of the structure, behaviour and life processes of organisms such as plants, animals and human beings.
Biotechnology can be used to:
- develop new diagnostics, vaccines, and treatments to improve health
- lift agricultural productivity
- manage the environment through pollution and pest control and the use of organic materials to generate energy
- assist with industrial innovations.
1.1.1 The value of biotechnology
In addition to benefits from its social and environmental applications, biotechnology has the potential to generate great economic value. There are several steps to gaining these benefits-often described as a 'value chain'-shown by Figure 1A.
The biotechnology 'value chain'
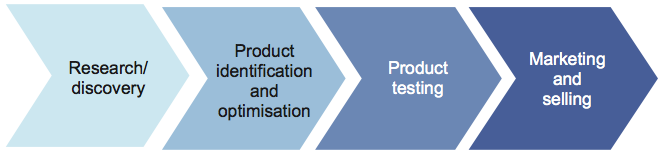
The further biotechnology initiatives and products go along the value chain, the greater the potential economic, environmental and social value to be gained. However, progress along the value chain is not easy or certain
- A new product can take a long time to develop. For example, Gardasil, the vaccine against the human papilloma virus, invented in Australia, took 19 years from research at the University of Queensland to the market.
- New products may require a high level of investment.The average cost, including the cost of failures, of taking a new drug from the laboratory to the pharmacist's shelf is estimated at US$800million. Smaller biotechnology companies, therefore, often need to partner with large firms during the process.
- Success is very rare. It is estimated that only one in every 1000 compounds identified as potential drugs make it to the market.
Although the biotechnology sector has great potential to generate private economic value, it has typically relied on public funding to 'seed' scientific and commercial developments. In many parts of the world the sector has received significant government investment.
The main argument for public funding stems from the perception of 'market failure' in the sector. This occurs when profit-seeking investors avoid funding research into possible new scientific products, due to the high risk of failure of experimental products, the difficulty of realising returns from successful products, and time taken to yield a final product.
The broader economic argument for public funding of biotechnology development is based on its potential to create benefits such as improved public health, increased social wealth, and more skilled employment.
1.1.2 Value of the life sciences industry sector
The Victorian Life Sciences 2009 Sector Report commissioned by the former Department of Innovation, Industry and Regional Development, now known as the Department of Business and Innovation (DBI), shows that at 30 September 2009 there were 44 publicly listed companies and about 90 private companies in the Victorian biotechnology sector.
Of the 44 publicly listed companies, only 13 were profitable. The sector is dominated by one of the top ten global biopharmaceutical companies, which accounted for 83 per cent of the $23.8 billion combined market capitalisation of the Victorian life sciences industry sector in 2009.
This one company's profit of $1.145 billion for the 2009-10 financial year largely offset the losses for the rest of the listed biotechnology companies in Victoria, giving the sector an aggregate net profit of $981 million. This configuration of the sector is linked to the case for government intervention.
1.2 Biotechnology policy and strategy
1.2.1 Encouraging innovation
Since 1999, DBI has funded $3.442 billion on programs and investments in science, technology and innovation (STI) to boost Victoria's science base. Many of these activities overlap, so DBI does not try to determine exactly how much it has spent specifically on biotechnology.
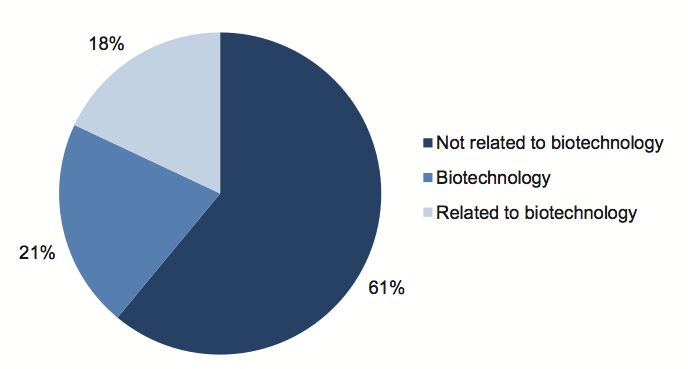
DBI estimates that, since 1999, of the $3.442 billion spent on STI initiatives, $722 million (21 per cent) has been directly spent on biotechnology, and another $610 million (18 per cent) has been indirectly spent in areas that have some relationship to biotechnology as shown in Figure 1B.
Figure 1B
Victoria's $3.442 billion innovation spending 1999-2009
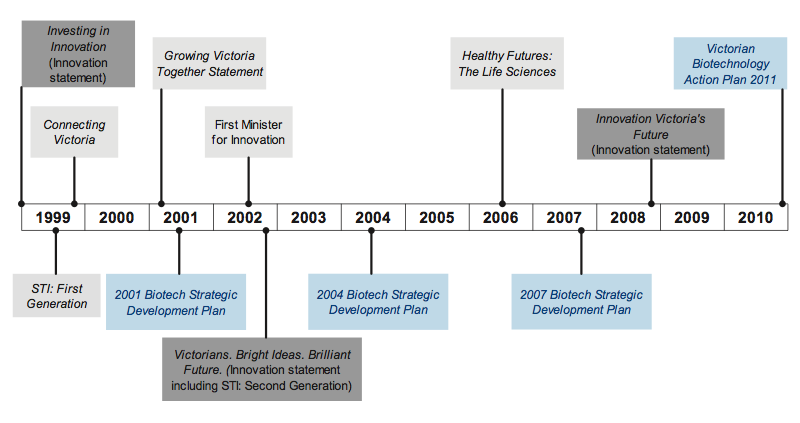
Since 1999 there have been various innovation policies, strategies and activities, as shown in Figure 1C.
Figure 1C Biotechnology and innovation strategies
1.2.2 The biotechnology strategic development plans
The 2001 Biotechnology Strategic Development Plan for Victoria (BSDP) envisioned that 'by 2010 Victoria is recognised as one of the world's top five biotechnology locations for the vibrancy of its industry and quality of research'.
It aimed to strengthen the state's research and development base by investing significantly in building infrastructure and scientific capability. Two more BSDPs were published in 2004 and 2007.
The 2004 BSDP focused on 'co-investing' in facilities that would help the industry develop products to the pre-market stage. It aimed to attract investment capital, facilitate international partnerships and build capability in pilot-scale manufacturing and early product testing.
The 2007 BSDP proposed a framework to build a mature and commercially sustainable sector by 2010 by using a partnership model between government, industry and research organisations to attract philanthropic and Commonwealth co-funding.
The most recent, the Victorian Biotechnology Action Plan 2011 was released just before the state election in November 2010. However, at the time of this audit the new government had yet to release any specific biotechnology policy or confirm whether it would support the action plan.
DBI is responsible for producing the BSDPs and the strategic coordination of the state's biotechnology. Between 2001 and 2007, the government allocated $49.6 million to DBI to develop and implement the BSDPs.
1.3 Direct public sector biotechnology investment
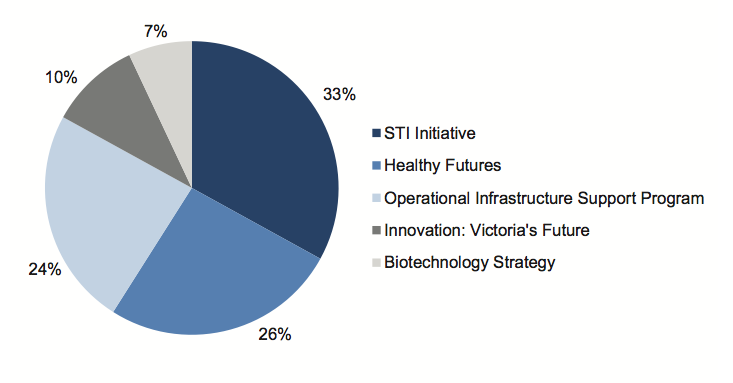
Figure 1D shows the breakdown of the $722 million direct public sector investment in biotechnology by program source.
Figure 1D
Victoria's $722 million in biotechnology funding 1999-2009
cite>Source: The Department of Business and Innovation.
The funding sources shown in Figure 1D comprise:
- Science Technology and Innovation Initiative: First and Second Generation-supports biomedical, environmental, agricultural, manufacturing, design, and information and communication technologies projects in metropolitan and provincial Victoria with competitive grants and allocated funding
- Healthy Futures-aims to build infrastructure and skills in the medical research sector and to expand ability to make medical breakthroughs
- Operational Infrastructure Support Program-annual performance-based funding to independent medical research institutes for essential infrastructure not funded by research grants
- Innovation: Victoria's Future-a policy statement that promoted a 'healthy, sustainable, productive' Victoria
- Biotechnology Strategy-a program within DBI todevelop and coordinate the BSDPs. The strategy is aimed at addressing gaps in biotechnology infrastructure, skills and information.
Both the STI and Operational Infrastructure Support Program commenced before the release of the 2001 BSDP.
1.4 Audit rationale and objectives
Public investments in biotechnology are often seen as intrinsically 'good', because their ultimate purpose is to extend and to improve human life.
Nevertheless, they still require appropriate accountability and performance management scrutiny including examining their rationale, efficacy, benefits, and outcomes.
Although precise measurement can be challenging, due to the overlap of biotechnology with the innovation economy, it is reasonable to expect that:
- the potential benefit from each public investment is identified
- the methods chosen to achieve the benefit are reasonably based
- costs incurred are economical and give value-for-money in the context
- progress is monitored to assure that it is on the right pathway to achieve goals, particularly when these are hard to measure or will take some time to achieve
- the outcomes are measured to verify achievements and learn lessons that can assist future investment decisions.
1.4.1 Objective of this audit
The aim of this audit was to examine the efficiency and effectiveness of public sector investment in the biotechnology sector.
The three sub-objectives were to review whether:
- BSDPs have been effective in guiding public sector investment and developing successful biotechnology-based industries
- DBI has appropriate systems to manage investments and to enable assessment against objectives
- the public has received value-for-money and benefits have been measured and realised.
1.4.2 Audit approach
To address the objective the audit examined DBI's biotechnology related activity, including:
- program design
- program governance
- investment evaluations
- business case reviews
- progress reporting
- benefit assessments.
The audit also examined biotechnology related activities at:
- >Australian Synchrotron Company Limited
- Australian Synchrotron Holding Company Limited
- Bio21 Molecular Science and Biotechnology Institute (its parent institution, the University of Melbourne
1.4.3 Structure of this report
The report is structured as follows:
- Part 3 - Managing public sector biotechnology investments.
2 Biotechnology strategy
At a glance
Background
Since 2001 all three biotechnology strategic development plans (BSDP) have envisioned that 'by 2010 Victoria is recognised as one of the world's top five biotechnology locations'. The Department of Business and Innovation (DBI) is responsible for developing, monitoring and reporting on Victoria's whole-of-government biotechnology activities.Conclusions
Victoria has made evident progress in developing a successful biotechnology industry and research hub with investment largely consistent with international benchmarks and criteria. However, since no measures or targets have been established to compare with other leading international biotechnology locations, it is not possible to conclude whether Victoria is or will be 'one of the world's top five biotechnology locations'.
There has been no overarching strategy to guide biotechnology activities, nor the $722 million of direct investment, creating the potential for both duplication and gaps in investment.
DBI cannot demonstrate a cause and effect link between its activities in the biotechnology sector and the results achieved. It is, however, reasonable to attribute at least part of the achievement of BSDP targets to investments made by the public sector.
Recommendation
DBI should develop an overarching biotechnology strategy to:
- guide whole-of-government activities and investments in the sector
- link the various funding programs to optimise investments' contribution to the agreed goals and targets for the sector
- set cause and effect measurement criteria that will allow for progressively more objective assessment of the achievement of goals and strategies.
2.1 Introduction
In 2001, the first Biotechnology Strategic Development Plan for Victoria (BSDP) was launched, with a vision that 'by 2010 Victoria is recognised as one of the world's top five biotechnology locations for the vibrancy of its industry and quality of research'.
The former Department of Innovation, Industry and Regional Development, now known as the Department of Business and Innovation (DBI), developed three BSDPs. It received $49.6 million between 2001 and 2007 to coordinate this process as well as to monitor and report on whole-of-government biotechnology activities. This monitoring role applies to about $722 million in direct public sector investment in biotechnology.
The 2001 BSDP aimed to strengthen the biotechnology research and development base by investing in scientific infrastructure and capability. Subsequent BSDPs were developed in 2004 and 2007. This audit does not review the most recent plan, published in late 2010. DBI reports against BSDPs to government, the biotechnology sector and the public on whether it has met the goals and targets.
The specific targets and goals in these BSDPs have changed over time, however, the core aspirational goal for Victoria to be one of the world's top five biotechnology locations has been constant.
This Part of the report examines whether the BSDPs have been effective in:
- guiding public sector investment in biotechnology projects
- developing successful biotechnology-based industries in Victoria.
2.2 Conclusions
There is no overarching biotechnology strategy to guide all publicly funded biotechnology activities and investments. Although BSDPs have high-level goals, such as Victoria's place in biotechnology in the international context, they are a small part of overall biotechnology expenditure. Therefore, their ability to impact on the performance of overall sector investment is likely to be limited.
The various biotechnology projects, initiatives and funded activities are not coordinated or reviewed for potential synergies. This increases the likelihood of inappropriate prioritisation and funding, and could lead to duplication, inefficiency and waste. Such outcomes would reduce the state's ability to gain optimum value-for-money from its investments.
DBI has reported that 10 of the 16 high-level strategic goals and targets set in the three BSDPs for biotechnology over the last decade have been met. However, DBI cannot demonstrate that the funded programs and activities achieved those results. This is because the measures adopted by DBI do not clearly demonstrate how the $722 million contributes to meeting the strategic goals and targets for biotechnology.
Because there are no measures or targets for benchmarks, it is not possible to determine whether Victoria is on the way to, or perhaps has already achieved, its goal of being one of the world's top five biotechnology locations.
2.3 Coordinating public sector investments
The five main policies and programs that fund biotechnology are:
- Science Technology and Innovation Initiative: First and Second Generation-since 1999 $638 million of funding through competitive and non-competitive rounds to 136 investments, most of it for infrastructure.
- Healthy Futures-building infrastructure and skills in medical research. It was launched in 2006 with $230 million to support growth in medical research and the life sciences.
- Operational Infrastructure Support Program-annual performance-based funding to independent medical research institutes to support indirect costs associated with carrying out research which are not specifically funded by government research grants.
- Innovation: Victoria's Future-commits a further $300 million to innovation initiatives including: $145 million for Victoria's Science Agenda, $50 million for the Victorian Life Sciences Computation Initiative and $20 million for 'Biotechnology Bridges'.
- Biotechnology Strategy-small grants, usually a few hundred thousand dollars, to a range of activities, the majority of which are related to marketing.
These policies and programs account for over $1.2 billion of the state's investment in biotechnology and related projects.
Figure 2A outlines the share of the programs spent directly on biotechnology projects and initiatives.
Most of the funding in Figure 2A has been for infrastructure, skills and basic research and development, with BSDP funded programs contributing only 7 per cent of the total.
Figure 2A
Public sector investment in biotechnology since 1999
|
Policies and programs |
Focus of funding |
Recipients |
Biotechnology investment ($mil) |
|---|---|---|---|
|
Science Technology and Innovation Initiative: First and Second Generation |
Infrastructure research and development |
Universities, research organisations |
243 |
|
Healthy Futures |
Infrastructure research and development |
Universities, research organisations |
185 |
|
Operational Infrastructure Support Program |
Infrastructure |
Medical research institutes |
174 |
|
Innovation: Victoria's Future |
Infrastructure |
Universities, research organisations |
70 |
|
BSDPs |
Industry development |
Industry, research organisations |
50 |
|
Total |
722 |
||
Source: Victorian Auditor-General's Office.
Criteria for allocating funding under the programs do not specifically assess whether the projects align with the strategic goals and targets in the BSDPs.
For example, they do not require an assessment of whether the project being considered will help Victoria achieve the goal of being one of the world's top five biotechnology locations.
2.4 Biotechnology strategic development plans
The BSDPs articulate the state's vision for biotechnology. The 2001 BSDP vision was to 'strengthen the research and development base by investing significantly in building up infrastructure and scientific capability'. DBI has stated that the BSDPs are 'industry development frameworks' and should not be used as overarching strategies to coordinate the many different public sector investments made in the biotechnology sector. The relatively low budget attached to them along with the specific actions tends to support this view, however, this also highlights the lack of a single strategy to guide biotechnology investment by the state.
In summary:
- the 2001 BSDP-set out the fundamentals needed to build a successful biotechnology sector, including strengthening research and development (R&D) foundations and facilitating industry growth through start-up companies, R&D partnerships and clinical trials
- the 2004 BSDP-focused on building international alliances and filling gaps in the discovery-to-market pipeline, with the aim of building a critical mass of infrastructure, people and companies in Victoria's areas of biotechnology excellence
- the 2007 BSDP-reflected industry changes over the preceding three years and adopted an outcome-focused and partnership approach. The plan focused on building substantial and sustainable firms, forging closer collaboration between researchers and firms, and integrating developments in biotechnology into the state economy.
Formal reporting of progress against targets takes place at the end of each of the BSDP cycles. The latest progress report, Victorian Biotechnology Strategic Development Plan 2007 - Towards 2010: Year 2 Progress Report , published in March 2010 stated that of the five targets for 2010, two had been met, and the other three were on track for the end of 2010.
The Victorian Biotechnology Action Plan 2011, released just before the state election in November 2010, was recently removed from departmental websites. At the time of this report the new government had yet to release any specific biotechnology policy.
According to DBI, the department is preparing advice for the government on development of a new biotechnology industry development plan.
Figure 2B sets out DBI's assessment of achievements against the targets from the 2001, 2004 and 2007 BSDPs. Apart from the 'top five' goal there is only one consistent target for all three iterations of the BSDP. There is no evident connection between investment activity and the targets.
Figure 2B Progress
against BSDP targets as assessed by the Department of Business and Innovation
|
Targets |
DBI assessment |
Target retained |
|
|---|---|---|---|
| Achieved |
In progress |
||
| 2001 BSDP | |||
|
Start-up companies -50 new start-up companies by 2005 |
Yes |
No |
|
|
Research and investment partnerships -at least five new research or investment partnerships with local or international biotechnology related companies by 2005 with a combined project value of $25 million |
Yes |
Yes |
|
|
Clinical trials -a 50 per cent increase in clinical trial research investment by 2005 |
Yes |
No |
|
|
Manufacturing -the creation of three significant manufacturing facilities by 2010 |
Yes |
No |
|
|
Community information -community groups feel well informed and involved in the policy development processes |
Yes |
No |
|
| 2004 BSDP | |||
|
Venture capital -venture capital investment in biotechnology to exceed 40 per cent of the national annual venture biotechnology investment by 2007 |
Yes |
No |
|
|
Partnerships -deals with a combined project value to exceed $1 billion in aggregate by 2007 |
Yes | Yes | |
|
Research and development -corporate biotechnology R&D expenditure to exceed $500 million per annum by 2007 |
Yes |
No |
|
|
International leadership -Victoria is recognised internationally as the leading location for marsupial genomics by 2007 |
Yes | No | |
|
Infrastructure -establishment of additional bioprocessing facilities for firms and the research sector by 2007 |
Yes | No | |
|
Patents -by 2007, over 120 US biotechnology patents per annum granted from the R&D base |
Yes | No | |
| 2007 BSDP | |||
|
10 firms will have market capitalisation of $250 million |
Yes | No | |
|
$1 billion of capital raised by the biotechnology sector |
Yes | No | |
|
Two major cross-sectoral projects initiated that address climate change through biotechnology applications in agriculture and industry |
Yes | No | |
|
Five new significant international projects facilitated |
Yes | Yes | |
|
Victoria will have established a biomarker system with the capability to adopt new technology in an ethical and equitable framework |
Yes | No | |
Source: Victorian Auditor-General's Office.
Many targets are at a macro level and are subject to factors beyond the government's control. There is no overt connection between investment activity and the targets defined. It is, therefore, difficult for DBI to demonstrate specific achievements from particular investments against the targets.
The nature of investment in innovation means that it is hard to attribute achievements to the investment intervention. During this audit DBI was unable to demonstrate clear causal links between its investments and the results achieved in the biotechnology sector. However, it is reasonable to attribute a degree of causality if the case for the investment was well developed and informed by best practice in innovation investment.
2.4.1 Investment logic mapping for biotechnology
In February 2010 DBI commissioned 'an independent review of the role of government in the development of the biotechnology sector to inform the development of its 2010 Biotechnology Action Plan. This review confirms DBI's current approach, which prefers robust program logic to support industry-based programs with an overarching strategic policy framework that seeks to support the environment within which industry can operate'.
The advisory report adopts the Department of Treasury and Finance's Investment Lifecycle Guidance approach. The report produces a preliminary investment logic map, which helps to define in diagrammatic form problems, interventions, benefits and key performance indicators for the biotechnology sector, as shown in Figure 2C.
This investment logic map will be a useful tool to help DBI clarify and define the biotechnology sector's service need, or in other words, why the government should intervene or invest in the sector.
Having a clear understanding of the need to invest or intervene will be complemented by DBI's assessment and analysis of the level of risk related to the various intervention or investment choices available for the biotechnology sector portfolio. A potential approach for DBI to understand these risks at a portfolio level is discussed in more detail in Part 3 of this report.
Figure 2C
Preliminary investment logic map for the 2011-14 BSDP
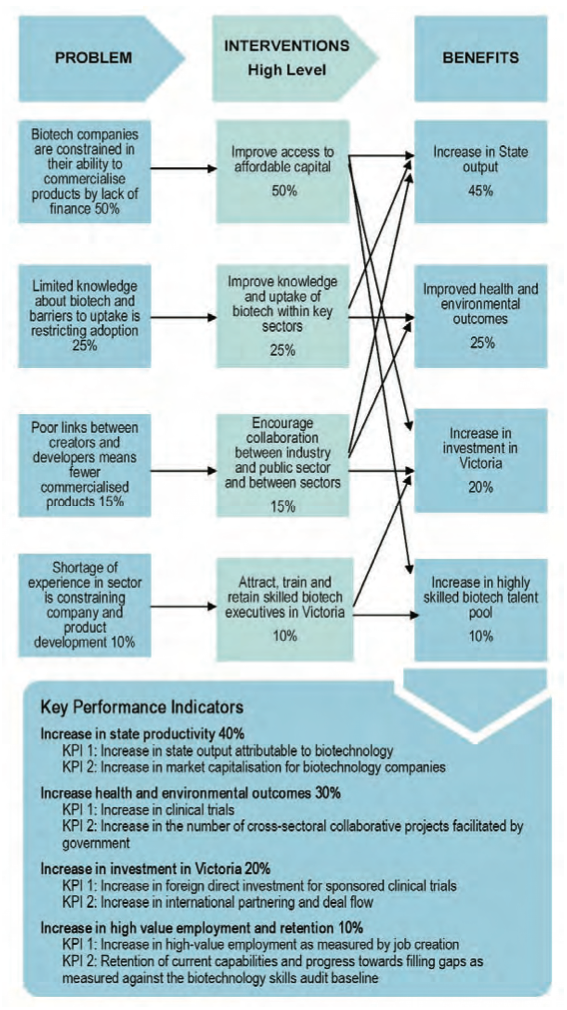
Source: Department of Innovation, Industry and Regional Development Strategic Assessment for the Biotechnology Strategic Development Plan 2011-2014, KPMG, May 2010, p.12.
2.4.2 The 'top five' global biotechnology location target
Since 2001 there has been a consistent BSDP vision for the sector to reach a 'top five' global ranking position. However, the BSDPs do not explain how Victoria's world ranking will be assessed and there is currently no agreed measurement system.
Apart from the absence of published measures of what 'top five' means, there are other difficulties in meeting this aspirational goal. Other jurisdictions around the world spend significantly more than Victoria, and have greater investment in their biotechnology sectors. This places the state at a comparative disadvantage.
Biotechnology is an enabling technology with medical, industrial, agricultural and marine applications. Victoria is not consistently strong across all of these areas. For example the 2007 BSDP makes no mention of marine biotechnology. Since the first BSDP in 2001, DBI has not explained how it will benchmark Victoria's international ranking and independently validate it.
It is important to set these measurement criteria transparently, rigorously and early so that the benchmarking verifies Victoria's performance against other international locations.
Until DBI develops this benchmarking detail, it is hard to conclude definitively on progress towards the goal that Victoria will be one of the world's top five biotechnology locations.
Recommendation
- The Department of Business and Innovation should develop an overarching biotechnology strategy to:
- guide whole-of-government activities and investments in the sector
- link the various funding programs to optimise investments' contribution to the agreed goals and targets for the sector
- set cause and effect measurement criteria that will allow for progressively more objective assessment of the achievement of goals and strategies.
3 Managing public sector biotechnology investments
At a glance
Background
Over the past decade, the Department of Business and Innovation (DBI) has spent $722 million to support the Victorian biotechnology sector.
Conclusions
Since 1999, DBI funding has been effective in assisting the delivery of a range of world class facilities and technology platforms for the biotechnology and broader life sciences sector.
Although the investments examined have the potential to generate a high return on investment, DBI cannot reliably demonstrate whether benefits have been achieved as a consequence of the investments made.
Findings
- DBI does not use recognised methodologies to determine portfolio risk and inform investment allocations. Consequently investment planning and strategic option analysis for potential investments cannot be assured as comprehensive and rigorous.
- DBI is unable to reliably and consistently demonstrate achievement of benefits as the approach to benefits measurement and management is not comprehensive.
Recommendations
setting, for each of the investments that it funds, performance targets that relate to strategic objectives
- DBI should adapt an investment portfolio allocation model to determine whether there is alignment of investments in the biotechnology sector with DBI's desired risk and return profile.
- DBI should further develop its grant management processes for biotechnology investments by:
- setting, for each of the investments that it funds, performance targets that relate to strategic objectives
- validating information provided by grant recipients
- embedding benefits planning in the funding approval process.
3.1 Introduction
Biotechnology is an inherently risky venture due to the high cost of commercialisation of new products and the high chance of once-promising discoveries being abandoned after further testing.
Given that there are usually many projects competing for limited public funding, if the Department of Business and Innovation (DBI) wants to be a strategic investor it needs to reliably determine which projects are more likely to realise the greatest benefits to the state.
To maximise the impact of public funds, project investments should therefore have a high likelihood of generating benefits. Thus, it is critical that inherent risks and expected benefits from proposed biotechnology projects are well understood by DBI. Measuring and reporting benefits from expenditure are key components in the chain of public sector accountability. These expectations are in line with global better practice for innovation programs, including the OECD's latest guidance, which advocates a mixture of high level performance measures, linked to attributable data from individual investments.
3.1.1 Scope of the audit and assessment criteria
This Part of the report examines DBI's approach to managing biotechnology investments. It relies upon detailed case study analysis to assess how DBI:
- makes funding decisions and manages risk
- implements and monitors the progress of funded projects
- measures benefits from biotechnology investments.
Figure 3A
Selected case studies of biotechnology investments
|
Project |
Funding received via policy/program |
Amount ($mil) |
|---|---|---|
|
Australian Synchrotron (a) |
Total state funding |
207.2 |
|
Bio21 Molecular Science and Biotechnology Institute (Bio21 Institute) (b) |
Science Technology Innovation (STI): 1st Generation |
30.0 |
|
Neurosciences Victoria |
STI: 1st Generation |
13.3 |
|
BioGrid (formerly Molecular Medicine Informatics Model) |
Healthy Futures: Victorian Life Sciences Statement |
12.6 |
|
Victorian Tissue Bank Initiative/Victorian Cancer Biobank |
STI: 2nd Generation |
7.0 |
|
Australian Tissue Engineering Centre |
STI: 2nd Generation |
5.2 |
|
Victorian Microarray Technology Consortium |
STI: 1st Generation |
4.4 |
|
Centre for Drug Candidate Optimisation |
STI: 1st Generation |
4.0 |
|
Total |
283.7 |
|
Note: (a) Comprises $100 million initial allocation, an additional $57.2 million STI 2nd generation funding and $50 million operational funding. (b) Includes crown land valued at $15 million.
Source: Victorian Auditor-General's Office.The case studies shown in Figure 3A were selected to represent the broad range of public sector funded activities and investments in the biotechnology sector. They were all funded in the period 2000-06 under various programs and were expected to deliver a range of outputs including physical infrastructure, technology platforms and research capability.
They account for 11 per cent of the public sector money identified as being spent on biotechnology since 2000.
To assess whether benefits from these investments were realised we examined whether:
- expected benefits were clearly articulated, realistic and measurable
- actual benefits were achieved
- benefit management plans were documented and included measures to monitor, analyse and report progress
- post-implementation reviews were conducted to assess if the business cases were realistic and to what extent the expected benefits have been achieved.
3.2 Conclusions
Since 1999, DBI funding has been effective in assisting the delivery of a range of world class facilities and technology platforms for the biotechnology and broader life sciences sector. This was confirmed through our examination of a range of investments in the sector.
However this examination also identified consistent and systemic issues in relation to planning for and measuring of benefits.
These issues can be summarised as follows:
- Portfolio risk analysis is not used to inform investment allocations. While selected investments considered in this audit were all assessed as high return, more than 82 per cent were also assessed as high risk.
- DBI's monitoring and evaluation of benefits is not sufficient.
- Benefits from investments are not always sustainable.
- DBI doesn't consistently know whether benefits have been achieved.
3.2.1 Portfolio risk analysis to inform investment choices
DBI does not use recognised and accepted methodologies to determine portfolio risk and inform investment allocations. This could lead to ad hoc investment planning and decision-making processes and limited strategic option analysis for potential investments.
3.2.2 Monitoring and evaluation of benefits
Because DBI's benefits measurement and management framework is not comprehensive or robust, DBI is not able to consistently demonstrate whether benefits have been achieved from public sector investments in biotechnology.
In particular the following weaknesses with benefits planning were evident:
- consistent criteria are not used for the selection of public investments due to the mix of funding types
- benefits planning is not consistently embedded into grants allocation or decision-making processes
- benefits are often poorly specified, with many difficult to measure or unrealistic benefits specified, and with some benefits set at too high a level and therefore unable to be convincingly attributed to the grant or project.
3.2.3 Sustainability of realised benefits
Notwithstanding the benefits which DBI report they have achieved, DBI's preferred funding models do not encourage identification and pursuit of sustainable future benefits.
This is because:
- most grants are one-off or too short-term in nature for benefits to have emerged during the grant period
- grantees are not required to develop an effective third party access or ownership strategy for department-funded scientific infrastructure after the grant term expires
- some of the funding models that DBI actively encourages are based on an overly optimistic assumption that the grantee will be able to generate sustainable fee-for-service revenue after the grant expires.
3.2.4 Achievement of benefits
In terms of benefits measurement, the following weaknesses were evident:
- reporting is at a very high level and is often based on grantee data that has not been validated
- a baseline of a current or 'before' state is not set, so that the 'after' state can be accurately measured
- data collected by DBI has been focused on sector-level metrics that do not measure the efficacy of individual grants
- retrospective studies commissioned by DBI have methodological weaknesses in that they use general economic activity as a proxy for specific public sector investment effectiveness.
3.3 Funding decisions and investment risks
The biotechnology industry sector is diverse and complex. This diversity and complexity increases the potential for public money to be wasted if the relationship between a proposed project's inherent risks, and the expected benefits, aren't appropriately defined and adequately considered when funding decisions are made.
3.3.1 Portfolio allocation and risk models
Portfolio allocation models are a common tool used by investors to understand the weighting of aspects of an investment portfolio. Qualitative and quantitative factors are used to assess the risk and return of proposed investments up front. This allows an investor to assess the investment options in the context of their risk appetite and likely return on their investment.
Portfolio management goals can be represented graphically, with the risk profile and potential value of investments assessed and plotted on a portfolio allocation graph. Public sector investors could adapt this model to suit their investment context, as shown in Figure 3B.
Figure 3B
A generic portfolio allocation model for public sector investors
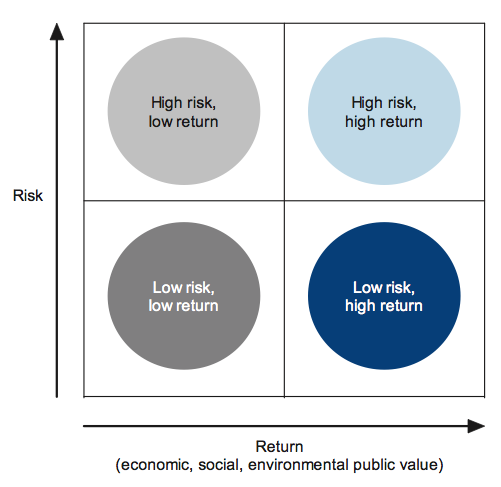
Source: Victorian Auditor-General's Office.
Biotechnology projects are inherently risky and costly and can take some time to show a return on investment, if at all. They may not achieve expected benefits despite a high level of investment. Consequently, private sector investors may be unwilling to invest in such projects. Economists describe this problem as 'market failure'.
Policies and programs administered by DBI can provide stimulus to the biotechnology industry market by providing grant funding, which eliminates the need for project proponents to raise costly private sector capital. Economists broadly describe this activity as 'market intervention'.
Unlike a profit-seeking investor, decision-makers in the public sector are not usually driven by a primary desire to receive a financial return on outlays. For example, in the case of biotechnology, development of the sector, and generation of 'public value' may be the key desired returns on public sector investment.
Notwithstanding that DBI may be less focused on making profits or monetary returns, it has a general duty to invest wisely and prudently so that value-for-money is optimised for the Victorian community. To do this, it needs to be particularly conscious and careful in its investment choices, as applicants for its grant funding are likely, to be largely from the high risk and high return quadrant.
This is because the logic of profit seeking private sector investors would lead them to focus the bulk of their investments in the low risk and high return quadrant, with fewer in the high risk and high return quadrant. The remaining two quadrants, which offer only low returns, would typically be avoided by investors from both sectors.
In order to illustrate how the portfolio allocation model could be used by DBI, Figure 3C shows our assessment of the risk-return ratio and relative cost of the eight representative investments we examined for this audit.
The size of each 'bubble' is proportional to the amount of investment, and the position of the bubble in a particular quadrant indicates our assessed level of risk and return for that investment.
Figure 3C
Risk and return ratio of selected biotechnology investments
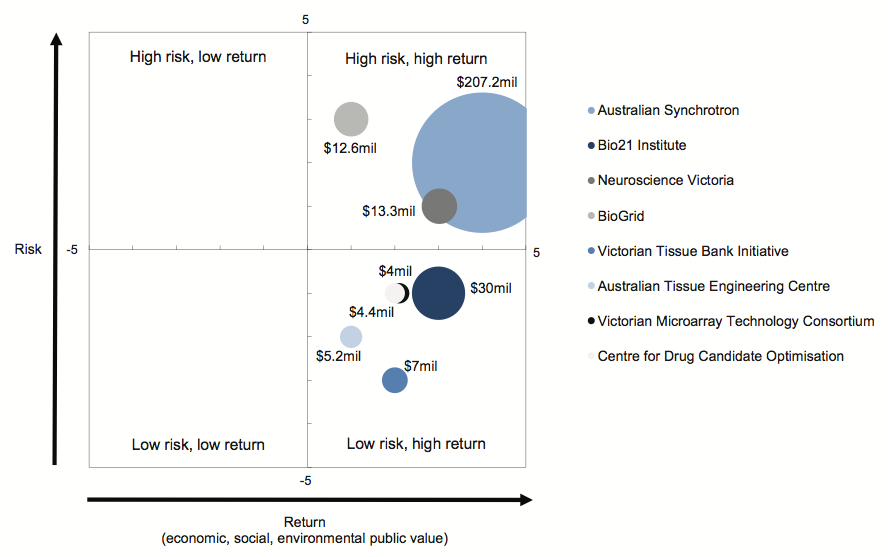
Source: Victorian Auditor-General's Office.
Figure 3C shows that the bulk of the value of the selected investments, $233.1 million (82 per cent), is in the high risk and high return quadrant. Although this allocation pattern shows a potential over-weighting of high risk investments, it also shows that all of the eight investments are expected to generate a high return from public investment.
Figures 3B and 3C highlight the potential usefulness of this approach for DBI, as the key public investor in the biotechnology sector, as it clearly maps the:
- relative cost of each investment in the portfolio
- assessed risk of the investment
- expected return from the investment.
3.3.2 Risk analysis for investment choices
It was not evident that an investment portfolio allocation model, or a reasonable alternative, is in place within DBI to inform its funding decisions.
DBI should consider adapting such a model to help structure its understanding of its historical and future investment portfolio. DBI should also use the model to determine whether there is alignment of investments in the biotechnology sector with DBI's desired risk and return profile.
3.4 Implementing and monitoring investments
Over the past decade, DBI has used both recurrent and capital asset funding of $722 million to support the biotechnology sector. The recurrent funding has mainly been in the form of grants which are managed according to DBI's internal financial and administrative processes.
Due to the mix of funding types used by DBI to support the biotechnology sector, there is a blurring of the concept of an 'investment' into the sector, as most of the funds have been disbursed via 'grants'.
However, some of DBI's grants to the biotechnology sector are more akin to an asset investment, due to the expectation that certain tangible assets will be acquired by the grantee, such as a specialised laboratory or a scientific instrument.
Because the state does not necessarily own the asset at the end of the grant period, DBI's grant funding to the biotechnology sector does not meet the technical definition of asset funding. Consequently this does not attract the typical central agency scrutiny or mandatory processes that apply to asset funding, including benefits measurement.
This means that DBI does not consistently use investment management tools. It should maximise the use of tools such as the Department of Treasury and Finance's (DTF) Investment Lifecycle Guidance , to assist with the development of clear investment logic maps, business cases and benefits plans for candidate investments.
Figure 3D
Business cases for the Science Technology Innovation grants
The business plans of the five STI projects reviewed had a number of key elements of good practice, including consistently capturing both the quantifiable and unquantifiable characteristics of the project, describing the need for the project as an enabling technology for modern biotechnology, identifying key stakeholders and conforming to the requirements of the STI business plan guidance.
Business cases were regularly updated to reflect the changing internal and external environment.
However, performance indicators used by the grantees and DBI do not provide a robust view of the likely success of the selected STI biotechnology projects, nor are they helpful in monitoring progress towards benefits delivery. KPIs are not consistently quantifiable or measurable and no report comprehensively measures and monitors performance against the identified KPIs.
Grant payments are conditional on the achievement of milestones, which are not always consistent with the KPIs and are often focussed on project process steps rather than outputs and outcomes.
DBI's quarterly progress reports do not therefore accurately and completely capture actual project performance in terms of progress made towards achieving the planned outputs and outcomes.
Source: Victorian Auditor-General's Office.
The DTF investment management approach emphasises the importance of a well-constructed investment case and shifts the focus from monitoring project metrics to strategically managing the long-term realisation of benefits.
Much of DBI's investment (or grant giving) activity is characterised by being one-off and short term, and could be described as 'donor' rather than 'investor' behaviour. This is because funds are disbursed to generate activity in the sector, without necessarily proactively managing a return (be it economic, social, or environmental).
Although good project and contract management processes are important, DBI needs to display more investment management behaviours to assure the government that its grants are delivering expected returns for the public's investment, and are thus value-for-money.
3.4.1 Contract and grants management
Milestones contained within funding agreements are used by DBI to monitor project performance and trigger stage payments. These milestones do not always correlate with project KPIs and are more an indicator of project processes, rather than the likely success of benefits delivery.
Milestones are project management metrics and represent a project view rather than a strategic investment view. However, we also noted examples where payments were made, even when grantees had not achieved expected milestones.
Figure 3E
Monitoring performance of the BioGrid investment
VAGO's review of the BioGrid project found that BioGrid has not complied with a number of requirements of DBI's funding agreement such as the development of:
- annual business plans
- a sustainability plan
- a comprehensive risk management plan.
DBI did not enforce compliance with these requirements although it approved funding for the project and continued to provide funding even though the grantee did not adequately address these critical issues until well into the project's life.
BioGrid project's current financial sustainability plan relies on significant increases in non-government revenue streams but does not substantiate the figures. There has also been a lack of consistency in the activities undertaken by the grantee to address the sustainability issue.
The revised funding agreement for BioGrid introduced a number of new milestones, but quarterly progress reports to DBI have identified that the project missed or only partly achieved certain agreed milestones. However, DBI is unable to effectively enforce these revised milestones by withholding future funding.
Source: Victorian Auditor-General's Office.
3.4.2 Measurement and reporting on effectiveness
DBI does not consistently analyse the outcomes and benefits of individual projects against their original business cases. This is a missed opportunity to capture lessons learned and to inform future investment decisions.
STI grant recipients complete a self-assessment survey on an annual basis after the grant period has ended. These surveys are generic and DBI does not have a formal process to independently validate the information provided nor does it systematically collate responses into a 'lessons learned' document for grant recipients.
Consultants commissioned by DBI have also recognised this issue, with their reports including a disclaimer to the effect that the information they have relied upon has not been validated.
DBI needs to improve its grant management processes by developing, for each of the biotechnology projects that it funds, performance targets that relate to relevant program objectives, as well as validating information from grant recipients.
DBI has taken some actions to improve its grant management process under the more recent Victoria's Science Agenda (VSA) program. This includes amending grant guidelines, redesigning the outcome monitoring survey, and revising the grant database and grant funding agreement template.
3.4.3 Conclusion on effectiveness of processes
DBI's administrative structures are mainly focused on grant and contract compliance processes and do not provide useful performance data about whether the strategic (policy) intent of government is being delivered.
The focus of DBI's grant giving processes appears to be on short-term stimulatory activity in the sector, rather than on delivery of long-term strategic benefits.
3.5 Case studies: benefits realisation
3.5.1 Summary of findings
In summary, the benefits realisation results for the investments we reviewed have been mixed. Figure 3F provides our assessment against the assessment criteria for all the reviewed investments.
3.5.2 Detailed findings from our review
Appendices A and B provide the detail to support each assessment.
Figure 3F
Summary of findings for benefits realisation of selected biotechnology investments
|
Assessment criteria |
Australian Synchrotron |
Bio21 Institute |
Neurosciences Victoria |
BioGrid |
Victorian Tissue Bank Initiative |
Australian Tissue Engineering Centre |
Victorian Microarray Technology Consortium |
Centre for Drug Candidate Optimisation |
|---|---|---|---|---|---|---|---|---|
|
Expected benefits are clearly articulated, realistic and measurable. |
Some | Some | Some | Some | Some | Some | Some | Some |
|
Post-implementation reviews are conducted to assess if the business case was realistic and to what extent the expected benefits have been achieved. |
Most |
Some |
Most |
None |
Some | Some | Some | Some |
|
Actual benefits are identified (expected, unintended, and disbenefits) and are sustainable. |
Some | Some | Most | None | Most | Most | Most | Most |
|
Benefits management plans are documented and include KPIs which are used as the basis for monitoring, analysing and reporting progress. (a) |
None | None | None | None | None | None | None | None |
Note: (a) If no measurements have been defined for KPIs then the case study will not rank better than red. Most = Most of the attributes of good benefits planning practice. Some = Some of the attributes of good benefits planning practice. None = Does not display the attributes of good benefits planning practice.
Source: Victorian Auditor-General's Office.
3.6 Systemic issues from case study observations
Based on our review of eight representative investments in the biotechnology sector, there are a number of consistent thematic issues in relation to monitoring and evaluation and benefits realisation.
These issues can be summarised as follows:
- benefits realisation measurement is underdeveloped
- benefits from investments are not always sustainable
- DBI cannot systematically demonstrate whether benefits have been realised.
3.6.1 Benefits realisation measurement
Generally, DBI's measurement of activity in the biotechnology sector focuses on private investment activity. This is not directly attributable to DBI's own performance in the sector and is useful primarily as a way to understand the 'state of health' of biotechnology in Victoria.
One of these tools, the Victorian Life Sciences 2009 Sector Report, reports on:
- total market capitalisation of the Victorian listed life sciences sector
- number of life science companies in Victoria
- number of drug candidates in submission phase involving Victorian companies
- Victorian life science company listings
- sales of products by Victorian life science companies
- profit generated by the listed Victorian life science companies
- estimated employment created by the Victorian life science companies
- funding raised by listed Victorian life science companies
- employment and exports created by international life science companies in Victoria.
DBI has also developed the Outcome Monitoring Tool - STI monitoring survey (OMT) which uses standard questions to collect information on the achievements of STI projects in relation to the five core intended outcomes of the program.
The monitoring survey captures only limited information about the measured projects' performance and is primarily intended to provide information to reflect high level program performance in a relatively cost-efficient way.
The OMT does not distinguish outcomes from benefits, and confuses some outcomes with benefits. Since the OMT is a generic survey, the questions are not always relevant to a particular project, and therefore do not always link back to the expected benefits articulated in the project's business case. There may also be additional benefits identified in a project's business case that are not captured in the survey.
No accompanying documents substantiate the data reported in the monitoring survey. In theory, DBI requires its client managers to validate the data and to follow up with grant recipients if any discrepancies are noted. However, DBI could not provide any documentation of this validation process in relation to the selected STI projects reviewed in this audit. This means the accuracy and quality of the information collected through the monitoring survey is not assured.
None of the various reports or surveys we reviewed provide DBI with adequate information on progress made towards achieving the claimed benefits. As a result, DBI cannot be assured that project benefits either have been or will be delivered as proposed.
3.6.2 Benefits are not always sustainable
Since 1999, DBI funding has been effective in assisting the delivery of a range of world class facilities and technology platforms for the biotechnology and broader life sciences sector. This was confirmed through our examination of a range of investments in the sector.
These facilities have directly supported life sciences researchers and practitioners and have the potential to:
- stimulate scientific research and innovation
- assist pharmaceutical and medical discoveries
- attract more private investment
- develop highly skilled people
- extend existing scientific knowledge.
Many of the investments that DBI makes in the biotechnology sector are in the form of grants, which by their nature are a limited contractual relationship between DBI and the entity it funds.
DBI focuses extensive administrative effort on setting up and administering 'arms length' panels and advisors to help choose projects for funding. It also deploys numerous procedures and systems to manage contractual terms and milestones of funding agreements, and to acquit grant expenditure.
However, very little of DBI's up-front administrative effort is focused on making sure that the initiative that was funded-and any related benefits arising-are able to be sustained after the grant funding ceases.
This means that:
- most grants are one-off or too short-term in nature for benefits to emerge
- grantees are not required to develop an effective third-party access or ownership strategy for DBI-funded scientific infrastructure after the grant term expires
- some of the governance models that DBI actively encourages are based on an overly optimistic assumption that the grantee will be able to generate sustainable fee-for-service revenue after the grant expires.
The outcomes of DBI's biotechnology investments could be improved by testing some of the underlying assumptions used in grant decisions against known actual performance of similar investments over the past 10 years and applying these lessons to future investments.
3.6.3 Benefits realisation
Expected benefits (if defined) for DBI's investments in the biotechnology sector are usually documented in business plans written as a requirement of funding agreements. Our review of the selected investments showed that many purported benefits are more correctly described as outputs or activities.
Improved benefits planning and measurement by DBI would allow it to:
- embed benefits planning into grants allocation decision-making processes
- better specify benefits and map inter-relationships with other investments
- specify benefits more accurately, rather than being too high level, not measurable, or not realistic.
In terms of benefits measurement, we observed the following weaknesses:
- reporting is at a very high level and is based on data that has not been validated
- a baseline of a current or 'before' state is not set which means that the 'after' state cannot be accurately measured
- the data that is collected by DBI is focused on sector-level metrics which can't directly measure the efficacy of the grants
- retrospective studies commissioned by DBI have methodological weaknesses in that they use economic activity as a proxy for investment effectiveness.
Recommendations
- The Department of Business and Innovation should adapt an investment portfolio allocation model to determine whether there is alignment of investments in the biotechnology sector with the Department of Business and Innovation's desired risk and return profile.
-
The Department of Business and Innovation should further develop its grant management processes for biotechnology investments by:
- setting, for each of the investments that it funds, performance targets that relate to strategic objectives
- validating information provided by grant recipients
- embedding benefits planning in the funding approval process.
Appendix A. Benefits analysis of selected investments
Detailed findings for selected investments
This Appendix shows the detailed results of our reviews of the extent to which benefits have been realised for a number of key public sector investments in the biotechnology sector.
We examined:
- Australian Synchrotron-state funding of $207.2 million
- Bio21 Molecular Science and Biotechnology Institute-asset funding and Crown land contribution amounting to $30million.
We also examined Department of Business and Innovation (DBI) documents and processes related to the following investments:
- Neurosciences Victoria -grant funding of $13.34 million
- BioGrid (formerly Molecular Medicine Informatics Model)-grant funding of $12.6million
- Victorian Tissue Bank Initiative/Victorian Cancer Biobank -grant funding of $7million
- Australian Tissue Engineering Centre -grant funding of $5.2 million
- Victorian Microarray Technology Consortium -grant funding of $4.42 million
- Centre for Drug Candidate Optimisation -grant funding of $4 million.
To assess whether benefits from the above investments have been realised we examined whether:
- expected benefits were clearly articulated, realistic and measurable
- actual benefits were achieved
- benefit management plans were documented and included measures to monitor, analyse and report progress
- post-implementation reviews were conducted to assess if the business case was realistic and to what extent the expected benefits have been achieved.
The review relied upon the objectives set by government or grant funding agreements and are not an assessment of the overall performance of the recipient entity.
The Australian Synchrotron
Conclusion
The Australian Synchrotron has achieved benefits in line with the original expectations of the government when it agreed to invest in the facility.
However, DBI and the Australian Synchrotron should make an attempt to better articulate and measure these benefits, so that future funding after 2012 can be linked to more measurable and attributable outcomes.
Overview of the investment
A synchrotron is a powerful source of light that scientists can use to assess the structure of materials at a molecular level.
A 1999 study conducted for the government by a strategic economic centre at a Victorian university noted that 'The synchrotron will act as a catalyst for development: enabling technology transfer by bringing together researchers from industry and academia. It will be the focal point for the development of a high-technology industry cluster.'
In June 2001 the then government announced that the state would contribute up to $100 million towards the cost of building a synchrotron.
A further funding submission in January 2003 set out an enhanced design and additional funding requirement of up to $57.2 million. Construction commenced in September 2003 for a total supported cost of $157.2 million for the building and machine (excluding beam lines).
An additional $50 million was subsequently provided by the state to partially cover costs for the first five years of operation.
The Australian Synchrotron was officially opened in July 2007-on time and on budget-and has been operating as a functioning synchrotron since that date. Since its opening, the Australian Synchrotron has established itself as a world-class facility and one that should deliver long-term scientific, economic and social benefits to both Victoria and Australia.
The Australian Synchrotron has developed an investment case to seek future funding from the Australian and Victorian Governments beyond the current funding agreement which concludes at the end of the 2011-12 financial year. This is currently under consideration by the Victorian Government.
Expected benefits
While the estimated economic and social benefits contained in early studies were not subject to independent scrutiny, this would not have necessarily affected the underlying primary case for the investment as identified to the government.
The expectations contained in the 2003 funding submission to government provide a set of suitable key performance indicators (KPIs) that could be used by DBI or a third party for benefits measurement. Figure A1 assesses these KPIs against the Department of Treasury and Finance's (DTF) better practice guidance.
Figure A1
Assessment of Australian Synchrotron benefits against KPI criteria
|
Economic Development and Infrastructure Delivery Committee of Cabinet |
Meaningful |
Attributable |
Measurable |
|---|---|---|---|
|
Over the five-year construction period and its 25-year operating life, the synchrotron is expected to create up to 700 to 1 000 high-quality Victorian jobs |
Yes |
Yes |
Yes |
|
The potential creation of an R&D cluster around the synchrotron and induced economic benefits in user industries has the potential to create up to a further 1 500 jobs |
Yes |
Yes |
Yes |
|
An expanded pharmaceutical industry in Victoria building on research in rational drug design using the protein crystallography beamlines could create 1 500 jobs |
Yes |
Yes |
Yes |
|
The micro-manufacturing industry where, if Australia were to capture a share of the micro-manufacturing market in proportion to its share of total world production, 300 jobs would be created |
Yes |
Yes |
Yes |
|
Opportunities for spin off companies, and small to medium firms, to develop and market world class technologies using synchrotron technology |
Yes |
Yes |
No |
|
Opportunities for productivity improvements in areas such as mining production techniques, minerals analysis and environmental remediation |
Yes |
Yes |
No |
|
R&D support to improve Victoria's competitiveness in areas such as biotechnology, new materials development, agriculture and food technology |
Yes |
Yes |
No |
Source: Victorian Auditor-General's Office analysis of Department of Business and Innovation documents.
Identified benefits
A Gate 6 - Benefits Evaluation Gateway Review was conducted by DTF in September 2009. Its report stated that the Australian Synchrotron project objectives were:
- to be a national icon of leading-edge science, of pivotal importance within the research structure of Australia
- to boost economic development by increasing opportunities for innovation and commercialisation, and encouraging the clustering of research-based industries
- to be designed and maintained to satisfy at least 95 per cent of Australian synchrotron science requirements
- to be recognised within the international community of users as an advanced and well-managed facility that meets or exceeds national and international standards
- to be accessible and user-friendly to a wide array of users around Australia and the region, and to facilitate collaboration and multi-disciplinary research partnerships
- to be operated in a commercially responsible manner to ensure the facility can continue to grow and develop to meet the changing needs of the Australian research community through reinvestment.
Although these project objectives are not equivalent to specific benefits, they capture the general project benefits at a macro level.
The Gateway Review Report arrived at the same conclusion by noting that 'While the Review considered the initial objectives (as outlined in this report) to have been broadly met, there are no specific metrics attached to these objectives that would have allowed for a definitive assessment of the benefits achieved to date.'
Plans for benefits management
No benefits management plans were developed by DBI or the Australian Synchrotron to monitor, analyse and report the achievement of the project's expected scientific, economic and social benefits.
The benefits described in the 2003 funding submission were developed many years before the Department of Treasury published the Investment Management Standard-Benefit Definition. As such, there was no specific policy requirement at the time for DBI to develop a benefits management plan or a clearly articulated set of benefits or supporting KPIs.
However, the lack of a current Australian Synchrotron benefits management plan does not preclude DBI from determining at some future point the actual benefits delivered by the state's investment in the project.

An interior view of the Australian Synchrotron facility. Photo provided courtesy of the Department of Business and Innovation.
Post-implementation reviews
The Gateway Review Report made a range of favourable findings about the Australian Synchrotron investment including:
- 'The Australian Synchrotron [...] is operating at world-class standards for availability, beam line stability, safety and other key matters...'
- 'There was consistent feedback from national and international experts that the Australian Synchrotron represented a world-class outcome achieved at a 'bargain basement price', i.e., has provided good value for money.'
- 'The Review Team finds that the Australian Synchrotron was well conceived, effectively and efficiently delivered, has been successfully commissioned and is operating as a world-class facility.'
Based on our own observations and fieldwork, we agree with the observations of the Gateway Review team.
In terms of the sustainability of benefits, the Gateway Review Report noted a number of issues that could affect the ongoing sustainability of benefits. In particular, the report noted tensions between:
- staff of the Australian Synchrotron and its Board, arising from a culture clash between science and commercial governance
- the Australian Synchrotron and its members
- various governments with an interest in the facility.
However since these issues came to light, a new senior management team and new members of the Scientific Advisory Committee have been appointed. The Australian Synchrotron is also operating at full capacity.
Bio21 Molecular Science and Biotechnology Institute(Bio21 Institute)
Conclusion
The state's investment in the Bio21 Institute, through the University of Melbourne, has assisted the university in delivering and developing a high-quality facility that currently houses a number of collaborative research groups, major technology platforms and industry tenants.
Given the substantial investment of state funds in the Bio21 Institute, DBI should attempt to determine what benefits the Bio21 Institute has delivered.
Overview of the investment
The origin of the Bio21 Institute lies in a 1999 joint initiative between the University of Melbourne, the Walter and Eliza Hall Institute of Medical Research and the Royal Melbourne Hospital.
At that time, these three organisations secured offers of funding from the University of Melbourne ($50 million) and an anonymous donor ($30 million) whose offer was conditional on additional funding being obtained from the state government. The state government provided $50 million to the Bio21 Project, of which $30 million was invested directly in the Bio21 Institute facility (including a $15 million transfer in kind of Crown land).
The Bio21 Institute is an institute that forms one entity of the Bio21 Cluster and was a recipient of funds from the Bio21 Project for facility and capital investments.
The Bio21 Cluster is a loose grouping of biotechnology entities consisting of organisations and entities from academic institutions, private institutes and private sector companies.
The Bio21 Project was the vehicle used by the state and Bio21 Australia (originally Bio21 Corporate) to create the original Bio21 Cluster and manage approved Bio21-related investments in capital facilities. The majority of the state's investment in the Bio21 Project was allocated to the Bio21 Institute.
Given the absence of benefits directly attributable to the Bio21 Institute in the early stages of the project, we assessed the economic and social benefits of the broader Bio21 Project so that the Bio21 Institute's contribution to project benefits could be determined.
The benefits for the Bio21 Project were first articulated publicly as state government policy in the Biotechnology Strategic Development Plan (BSDP) published in 2001.
The benefits included:
- creating up to 100 new biotechnology companies over the next ten years
- attracting new investment directly into the industry at a rate of at least $30 million per year
- generating thousands of new jobs each year, both directly at the Bio21 facilities and through companies feeding off its activities
- creating millions of dollars in high value added export income.
The Bio21 Institute building was originally intended to accommodate over 1 000 people in order to meet its twin objectives of collocation and commercialisation. However, the building plans were scaled back and around half of this number currently work in the Institute. The Bio21 Institute building opened in 2005.

The internal atrium of the Bio21 Institute building. Photo provided courtesy of the Department of Business and Innovation.
Expected benefits
The economic and social benefits contained in the BSDP 2001 provide a set of suitable measures which could be used to measure the benefits of the Bio21 Project. These benefits cannot be achieved by the Bio21 Institute in isolation.
Figure A2
Assessment of Bio21 Project expected benefits against KPI criteria
|
BSDP 2001 benefits |
Meaningful |
Attributable |
Measurable |
|---|---|---|---|
|
Create up to 100 new biotechnology companies over the next ten years |
Yes |
Yes |
Yes |
|
Attract new investment directly into the industry at a rate of at least $30 million per year |
Yes |
Yes |
Yes |
|
Generate thousands of new jobs each year, both directly at the Bio21 facilities and through companies feeding off its activities |
Yes |
Yes |
Yes |
|
Create millions of dollars in high value added export income. |
Yes |
Yes |
Yes |
Source: Victorian Auditor-General's Office.
The Bio21 Project Business Plan does not contain any clearly articulated benefits statements.
It does, however, contain 13 objectives, some of which could be used to develop benefits statements and KPIs. The Bio21 Institute Business Plan 2005-2014 does contain a well developed set of KPIs and these have been used by Bio21 Institute to report performance internally within the University of Melbourne.
A range of these KPIs could be used to develop an agreed set of benefits applicable to the Bio21 Institute.
Actual benefits
The Bio21 Institute has delivered a range of positive outcomes to Victoria. These include:
- world-class infrastructure that houses strategically important biotechnology platform technologies that can be readily accessed by academia, government research organisations and industry within the Parkville biotechnology precinct
- a new and innovative organisational research model based around multi-disciplinary research with a focus on innovative science discoveries in the areas of structural biology, chemical biology and nano-biotechnology
- the co-location of academics, a cooperative research centre and industry promotes multi-disciplinary research and collaborative activity.
The achievement of these outcomes and the resulting benefits has been made possible through the establishment of the Bio21 Institute.
Plans for benefits management
Commercialisation was one of the original key objectives of the Bio21 Project, however, the underlying financial analysis of commercial revenues was unrealistic and overoptimistic at the time. In 2002, the department agreed that the focus of the Bio21 Project should be on collaborative research.
However, DBI did not fully assess the implications of agreeing to change the focus from commercialisation to collaborative research, particularly given that commercialisation was the fundamental driving factor behind the state's decision to fund the Bio21 Project.
As a result, DBI did not provide comprehensive advice to the Minister prior to the approval of the Bio21 Project Business Plan. DBI could have exercised the state's rights in the funding agreement to require more detailed performance data against the measures contained in the business plan. Thus, an opportunity was lost to provide a suitable vehicle to report benefits-related data once the project had closed.
DBI has not taken any action to obtain more detailed benefits reporting on the Bio21 Institute and did not assess whether its investment in the Bio21 Institute would produce any more or fewer benefits than would have otherwise been achieved by undertaking an alternative investment.
Any such assessment should clearly differentiate between the benefits arising from the investments in the Bio21 Institute and other entities involved in the Bio21 Project.
Post-implementation reviews
Although there has been a broad review of the Bio21 concept, no independent review has been conducted to specifically assess achievements of the four key benefits espoused by the government (refer to Figure A2) when it funded the facility (i.e. hundreds of companies, millions in new investment, thousands of jobs, millions from exports).
DBI should sponsor reviews by appropriately qualified independent experts to confirm the economic and social benefits arising from the state's investment in the Bio21 Institute.
Neurosciences Victoria
Conclusion
The expected benefits of the project are articulated in the business plans, however, some expected benefits are hard to measure. Achievement of expected benefits cannot be accurately assessed because no baseline measurement has been provided.
Notwithstanding these measurement challenges and weaknesses, significant benefits have been identified as arising from the investment.
Overview of the investment
Neurosciences Victoria (NSV) has been allocated $13.34 million in instalments from the first generation STI Initiative via a contestable process since January 2001.
Neuroscience is the scientific study of the nervous system. It explains how behaviour is related to molecular, cellular, and electrical events in the brain. Application of neuroscience research includes designing devices and systems to be more human friendly, prevention of mental disabilities, and improved treatment of psychiatric disorders.
The NSV consortium aims to:
- support the development of neuroscience research discoveries
- provide infrastructure to translate successful research discoveries into commercial applications
- organise and focus neuroscience research and attract public and private investment partners
- acquire the physical infrastructure required to place Australia at the forefront of international neurosciences research.
Expected benefits
The project's business plan proposed that the project would establish a world-class neuroscience capability and provide three key benefits.
However, the business plan does not specify what measures should be adopted to assess the success of these benefits. Some claimed benefits are intangible in nature and thus likely to be difficult to measure.
Figure A3
Assessment of Neurosciences Victoria benefits against KPI criteria
|
Business plan |
Meaningful |
Attributable |
Measurable |
|---|---|---|---|
|
A single point of access to Australia's leading neuroscientists for external groups to access world class research capabilities. This includes marketing, bid participation, management of all aspects of contracts, IP and commercialisation management, identification and engagement of all relevant scientific specialists |
Yes |
Yes |
Yes |
|
Provision and appropriate access regimes to the key infrastructure platforms and expertise in the field of neuroscience. Each platform developed has extended or created new capabilities which will be accessible by the broader scientific and commercial community. For example, small to medium biotechnology groups will have access to an extensive set of platform technologies to support its research programs and local scientists will have a streamlined access regime to utilise state of the art technology and expertise in a cost efficient and timely manner |
Yes |
Yes |
No |
|
Creation of a coordinated and well resourced group to effectively exploit the combined national IP in the field of neuroscience |
Yes |
Yes |
Yes |
Source: Victorian Auditor-General's Office.
Actual benefits
NSV has achieved some expected benefits, including: coordinating research effort and infrastructure needs between consortium members; providing a single point of contact for potential sponsors; and generating intellectual property.
These expected benefits, however, have not been comprehensively reported or measured.
Plans for benefits management
There was no evidence of the existence or use of documented benefit management plans or any other equivalent documents by DBI.
Post-implementation reviews
The final report submitted to DBI and the STI outcome monitoring survey identified achievements of expected benefits, including coordinating research effort and infrastructure needs between consortium members, providing a single point of contact for potential sponsors, generating intellectual properties, and other key outcomes.
Notwithstanding this, expected benefits have not been fully reported or measured. For example, it has not been clearly reported how small to medium biotechnology groups have used the platform technologies to support their research programs, and how much time and cost has been saved by local scientists using the technologies and expertise provided by NSV.
BioGrid (formerly Molecular Medicine Informatics Model)
Conclusion
The project has attracted more than $6 million in grants over and above the original $17 million first provided by Bio21 Australia Ltd, the Commonwealth Department of Education Science and Technology, and DBI to establish the initiative.
Up to January 2010, BioGrid had earned almost $425 000 in revenue from non-government sources, mainly pharmaceutical companies. These commitments from outside organisations suggest that BioGrid has potential to become a valuable research and clinical tool.
Although commercial revenue is growing, it is doing so slowly, making BioGrid's future sustainability uncertain. Its 2010-11 business plan forecast a shortfall of $3.9 million for the next three financial years. Due to these circumstances, BioGrid made almost half of its staff redundant in 2010.
Neither individual researchers nor their institutions appear willing to pay enough for the availability of BioGrid to keep the system going.
In such circumstances DBI should assist BioGrid to identify and measure what actual benefits have been realised and what potential benefits will be realised by the project. The results could become either a strong argument for further support of the system, or an argument for reconsidering its future.
DBI also needs to revisit its one-off funding model and recognise the necessity to provide on-going funding under certain circumstances in order to achieve expected benefits or sustain realised benefits.
Overview of the investment
BioGrid is a hardware and software technology platform for life science research teams to access and share genetic and clinical research data across multiple organisations. It achieves this by allowing computers containing research and clinical data to be linked together in an ethically approved, secure and controlled way, using the internet.
The data is stored at multiple institutions and can be linked, queried, and analysed by authorised researchers using BioGrid's federated data integrator (FDI). The FDI does not store data; data is sent to researchers over secure networks once it has been de-identified. Researchers have used BioGrid in research projects in areas including oncology, diabetes, and neuroscience.
BioGrid has the potential to help scientists improve and accelerate their research work. Clinicians can also use BioGrid to improve clinical decision-making and the overall quality of healthcare.
Figure B4 provides the history and sources of funding for the BioGrid Project since 2003. The original name of the project was Molecular Medicine Informatics Model (MMIM), which was part of the broader Bio21 Project.
Figure A4
History and source of MMIM/BioGrid public funding
|
MMIM/BioGrid project phases |
Date |
Amount ($mil) |
Funding source |
|---|---|---|---|
|
Phase 1: Bio21 MMIM |
September 2003 |
1.6 |
DBI (via Bio21 Australia Limited) as an STI Initiative project |
|
Phase 2 |
August 2005 |
4.37 |
Commonwealth Department of Education, Science and Training (DEST) |
|
Phase 3: Australian Cancer Grid |
September 2006 |
11 |
DBI through the Healthy Futures program |
|
Total |
16.97 |
||
Source: Victorian Auditor-General's Office analysis of Department of Business and Innovation and BioGrid documents.
Expected benefits
Not all benefits that have been identified have been meaningful, attributable and measurable, as shown in Figure A5.
Figure A5
Assessment of MMIM/BioGrid benefits against KPI criteria
|
STI initiative funding application |
Meaningful |
Attributable |
Measurable |
|---|---|---|---|
|
Capacity to select patients for clinical trials based on their genetic profile increasing Melbourne's attraction for drug trials and therefore providing a mechanism for patients to access novel, effective, and often very expensive therapies at no cost |
Yes |
Yes |
Yes |
|
Avoids the duplication of expenditure through an objective of developing a generic model and sharing the learnings |
Yes |
Yes |
No |
|
Attract and retain leading researchers in Melbourne |
Yes |
No |
No |
|
Provide the capacity to identify target components of new diagnostic tools or to identify and validate novel therapeutic targets |
Yes |
Yes |
No |
|
Pre-symptomatic testing of patients and early intervention based on genotype data and consequently improved healthcare outcomes |
Yes |
Yes |
Yes |
|
Economic potential from the development of commercially exploitable IP [and] the attraction of investors, such as pharmaceutical companies, supporting clinical trials and technology development plus export opportunities |
Yes |
Yes |
Yes |
|
A dramatic reduction in health care costs based on the knowledge so obtained and extensive information integration |
Yes |
No |
No |
|
An enhanced ability to provide high quality health care services to both metropolitan, country, and remote communities, and to export these services to Asia and beyond |
Yes |
No |
No |
|
Transfer of information technology know-how among the Victorian clinical research community |
Yes |
Yes |
No |
|
Reduced research costs, resulting from the ease of obtaining information and testing hypotheses |
Yes |
Yes |
No |
Actual benefits
The expected benefits that were identified were generally not tracked to determine whether the actual benefits were realised.
DBI advised that the six-monthly Healthy Futures report was prepared by DBI in consultation with the grant recipient to brief Cabinet on the key achievements of the project. However, this mostly reports on achievements of outputs rather than benefits, and of the very few benefits described in the reports, none were measured.
Some benefits were realised that were not explicitly anticipated. Some patients have received benefits with respect to the clinical management of cancer patients from the Australian Cancer Grid (phase 3) that were not identified in the original proposal for Bio21 MMIM (phase 1). Further unexpected benefits arose from a study that identified the reasons that patients do not attend surgical or clinic appointments and developed measures that may improve patient attendance.
No disbenefits were identified but some high-level disbenefits may exist. There has not been any attempt to identify whether the benefits listed in the 2003 Bio21 MMIM funding application and the 2005 Australian Cancer Grid funding proposal were actually achieved. Without this information, it is possible that the money spent on the project by the department and publicly funded institutions could have been put to better use, and in this sense there may be disbenefits to patients and other stakeholders.
A recent DBI memo noted 'the inability to identify the value of BioGrid' almost seven years after the project began. It shows that there has been a lack of focus by the department to identify actual benefits resulting from the expenditure of public funds since the inception of the project in 2003.
Plans for benefits management
There was no evidence of the existence or use of documented benefit management plans or equivalent documents.
Post-implementation reviews
There was no evidence of any post-implementation reviews for the project. A technical evaluation was done for Phase 1 of the project - the 2005 Bio21 MMIM Evaluation Precedence Research - but it focused on functional project objectives, not wider benefits.
The sustainability of the project is currently in doubt, and the lack of documented benefits may be an impediment for the new BioGrid governance structure to gain additional funding from members, researchers, and other organisations and individuals.
Victorian Tissue Bank Initiative/Victorian Cancer Biobank
Conclusion
Achievements of expected benefits cannot be assessed accurately without describing how or when to measure the expected benefits, the specific targets, nor the baseline against which progress should be measured.
Delivery of the expected benefits was not specifically measured and reported.
Overview of the investment
The Victorian Cancer Biobank is a not-for-profit consortium of tissue banks. It aims to provide high quality clinically annotated biospecimens to the academic and commercial cancer research sectors.
The Victorian Cancer Biobank has been allocated $7 million in instalments in a contestable process under the second generation STI Initiative since October 2005.
A tissue bank is a temperature controlled facility for storing clinical tissue samples to be used in approved research. It provides researchers with access to annotated human tissue samples to conduct large scale studies. It plays a critical role in translating biomedical and clinical research to assist in the detection, prevention and treatment of cancer.
Expected benefits
The project's business plan describes the potential benefits that it would generate. Most of these benefits are meaningful and achievable.
Figure A6
Assessment of Victorian Tissue Bank Initiatives (Victorian Cancer Biobank) benefits against KPI criteria
|
Business plan |
Meaningful |
Attributable |
Measurable |
|---|---|---|---|
|
Seek to facilitate and expedite research aimed at alleviating cancer-related conditions. The VTBI has the potential to fast track a solution to some or all of the major cancers in the wider domestic and international population |
Yes |
Yes |
Yes |
|
Significantly enhance capacity for tissue-based cancer research by Victorian scientists |
Yes |
Yes |
Yes |
|
Increase the molecular pathology base in Victoria through the support of two accredited pathologists and four senior pathology trainees or fellows |
Yes |
Yes |
Yes |
|
Provide a means to integrate tissue-based molecular pathology research and services. The special services provided by VTBI will develop the skill base of research assistants with molecular and histopathology training. The DNA Bank and construction of tissue microarrays would provide unparalleled access to material for basic and translational research |
Yes |
Yes |
Yes |
|
The capacity and skills base provided by VTBI would fill an existing gap that is currently an impediment to Victoria becoming one of the top five biotech hubs in the world |
Yes |
No |
Yes |
|
Serve as a core resource from which to drive cancer research activities, which would result in great direct and indirect benefits to the Victorian academic, pharmaceutical and biotechnology research market segments. It provides a vital link between existing Victorian preclinical and clinical trials resources that directly test new interventions for cancer |
Yes |
Yes |
No |
|
Not only help to keep funds that would otherwise go overseas during the pre-clinical phase, but also enable Victorian academic institutions and companies to compete more effectively for national and international funding opportunities, and offer a significantly stronger value proposition to industry as a state in which to translate cancer research |
Yes |
No |
Yes |
|
An enhanced, more accurate and timely ability to identify candidates for clinical trials |
Yes |
Yes |
No |
|
Ability to move from Clinical trial recruitment to clinical trial selection of specific patients who will have a predicted higher pre-disposition to react to therapy tailored to specific genes |
Yes |
Yes |
Yes |
|
The ability to form correlations between diseases, genotypes, clinical data and patient demographics supporting the identification of candidate compounds and advancing the personalisation of treatments for patients with specific genes |
Yes |
Yes |
Yes |
|
Addressing ethics, privacy and data custodial responsibilities while enabling clinical research community |
Yes |
Yes |
Yes |
|
Results sharing and networking with other members of the Victorian clinical research community |
Yes |
Yes |
Yes |
|
Transfer of information technology know-how among the Victorian clinical research community |
Yes |
Yes |
Yes |
|
Research hypotheses can be quickly tested across large data sets |
Yes |
Yes |
Yes |
|
Reduced research costs, resulting from the ease of obtaining information and testing hypotheses |
Yes |
Yes |
Yes |
Actual benefits
Key outcomes in relation to skills base, commercial outcomes, scientific research, collaboration, and science awareness were identified in the STI outcome monitoring survey.
Benefits were identified, including:
- establishing tissue banks in large regional centres
- supporting a medical scientist and a full time pathology registrar trainee position as well as equipment and some operating funds
- providing support and training to the new staff in the area of molecular pathology.
- generating biospecimens from more than 300 donors per year
- increasing supply of skilled labour
- increasing competitive grants for cancer research through increased participation in multi-centre clinical trials
- enhancing translation of research to health outcomes
- improving quality in research outcomes
- increasing the number of clinical trials in Victoria
- forming new commercial and research partnerships and providing access to new technologies and processes.
Apart from the number of clinical trials that use the Biobank resource within Victoria, the number of applications received from research groups and the number of pathology trainees sponsored by Biobank, other claimed benefits have not been specifically measured in any detail.
There were four clinical research projects in Victoria using the Biobank resource by 2009, compared to the target of 15 set in the grant. The Biobank also reported better than required achievement for another grant target, with six medical graduates undergoing specialist pathology training, compared to the grant target of four.
Plans for benefits management
There was no evidence of the existence or use of documented benefit management plans or equivalent documents by DBI.
Post-implementation reviews
The final project report submitted to the department outlines key achievements of the project. However, most of the achievements were outcomes or outputs.
Although some social and economic benefits were identified, they were not specifically measured or quantified. Many expected benefits have not been tracked or measured by DBI.
Australian Tissue Engineering Centre
Conclusion
Achievements of expected benefits cannot be assessed accurately without describing how or when to measure the expected benefits, the specific targets, nor the baseline against which progress should be measured.
Delivery of expected benefits has not been specifically measured and reported.
Overview of the investment
The Australian Tissue Engineering Centre (ATEC) has been allocated $5.2 million in instalments in a contestable process under the Second Generation STI Initiative since January 2006.
ATEC was funded to establish a Good Laboratory Practice (GLP) drug and product testing service in Melbourne. GLP is an international quality standard awarded to laboratories that meet key organisational processes and conditions to a pre-determined standard.
It could attract potential national and international contracts and relationships by providing contract research and development and testing services to biotechnology research institutes and companies.
Expected benefits
The project's business plan describes the private and public benefits that would be created through the project.
Figure A7
Assessment of Australian Tissue Engineering Centre benefits against KPI criteria
|
Business plan |
Meaningful |
Attributable |
Measurable |
|---|---|---|---|
|
Fill the critical infrastructure gap for preclinical testing |
Yes |
Yes |
No |
|
Functioning as an incorporated company where funds, resources, infrastructure and IP is shared and maximally used |
Yes |
No |
No |
|
Attract private investment into R&D programs |
Yes |
Yes |
Yes |
|
Provide opportunities for research and commercial collaborations |
Yes |
Yes |
Yes |
|
Provide the necessary environment for high level specialised skill development and training |
Yes |
Yes |
No |
|
Create employment and research opportunities |
Yes |
Yes |
Yes |
|
Generate IP in the area of pre-clinical test modalities |
Yes |
Yes |
Yes |
|
Maintain the world-renown scientific pre-eminence of ATEC's members in TE and stem cell research |
Yes |
No |
No |
|
Attract industry aligned research and service contracts for associated industry and research institutes through the capability of the ATEC test modalities |
Yes |
No |
Yes |
|
Build critical mass for the Australian pharmaceutical industry |
Yes |
No |
No |
|
Generate human health and social benefits through the development of TE applications |
Yes |
Yes |
Yes |
|
Develop the necessary skills and infrastructure base for conducting research in new GLP TE tests |
Yes |
Yes |
Yes |
|
By providing GLP R&D expertise within Victoria, ATEC will keep potential drug development work in Victoria and channel work downstream to local services. It would then act as a funnel for the testing of lead materials and create economic benefits and employment for Victoria with the subsequent opportunity to undertake the full developmental testing through to clinical trials right here in Melbourne Leveraging existing, as yet unconnected benefits of stand-alone resources and facilities, so as to provide a holistic solution for the development of lead compounds |
Yes |
Yes |
Yes |
|
A NATA, Therapeutic Goods Administration (TGA) & Food and Drug Administration (FDA) approved GLP facility would provide the ability to compete for the $227 million of pre-clinical trial work currently leaving Australia to overseas organisation |
Yes |
Yes |
Yes |
|
ATEC would fill the gap and further attract academic and industry players to Victoria. Indirect benefits would include reversing an opportunity loss for capability building and attaining a critical mass of expertise that will be important in retaining existing skilled researchers and in attracting ex-pat researchers |
Yes |
No |
Yes |
|
ATEC would enhance Victoria's reputation as one the world's top 10 most investable biotech states in the world |
No |
No |
No |
|
ATEC could contribute to achieving the long term community goal of reducing the use of animals for drug testing |
Yes |
Yes |
Yes |
Source: Victorian Auditor-General's Office.
Many of these benefits are intangible in nature and difficult to measure. For those that are tangible and measurable, no specific measures have been described to assess the delivery of these expected benefits.
Actual benefits
Key outcomes in relation to skills base, commercial outcomes, scientific research, collaboration, and science awareness were identified in the STI outcome monitoring survey.
No other specific benefits have been reported.
Plans for benefits management
There was no evidence of the existence or use of documented benefit management plans or equivalent by DBI.
Post-implementation reviews
The STI monitoring surveys identified key outcomes of the project. No other reports provided a review of benefits which had already been realised and those to be realised.
The captured expected benefits include number of IP applications, employment created by ATEC, as well as information on training and skill development provided. However, other expected benefits have not been specifically measured or tracked by DBI.
Victorian Microarray Technology Consortium
Conclusion
Achievements of expected benefits cannot be accurately assessed without describing how or when to measure the expected benefits, and specifying targets, or the baseline against which progress should be measured.
Victorian Microarray Technology Consortium (VMTC) has reported to DBI that it is providing open access to the microarray technology platform and necessary training to academia and industry through clustering and collocation of equipment and expertise. VMTC staff provide expert advice in experimental design, technology development and applications with monitoring and supervision. The VMTC also offers high-level training in the platform technology that may lead to the growth in knowledge, general awareness in the scientific community and successful application of this technology.
VMTC has attracted $23.75 million to projects either using or requiring access to the platform technology.
However, delivery of expected benefits for the project has not been specifically measured and reported, with only some outcomes and limited benefits identified.
Overview of the investment
VMTC has been allocated $4.42 million in instalments from the first generation STI Initiative's contestable process since July 2001.
The vision of the VMTC is to establish microarray technology as a globally competitive and unique platform technology to help develop the biotechnology sector in the agrifood and biomedical fields in Victoria. It will also provide training in, and access to, this key platform technology.
Most cells in our bodies contain the same genes, however, only a fraction of the genes are turned on or 'expressed' in each cell to specify properties unique to each cell type. To understand how cells achieve such specialisation, scientists need a way to identify which genes are expressed by each cell type.
To date, scientists and researchers have only been able to survey a relatively small number of genes at a time by using traditional methods to analyse gene expression. Microarray technology provides a tool to help scientists and researchers examine and analyse expression of thousands of genes in a single experiment more quickly and efficiently.
Expected benefits
The project's business plan outlines private revenue and public value that can be generated through the project.
Figure A8
Assessment of Victorian Microarray Technology Consortium benefits against KPI criteria
|
Business plan |
Meaningful |
Attributable |
Measurable |
|---|---|---|---|
|
The project has the potential to develop new business opportunities in drug and pharmaceutical development and improved agricultural production and food processing |
Yes |
Yes |
Yes |
|
Formation of the Consortium maximises investment efficiency and reduces operating costs through clustering |
Yes |
Yes |
Yes |
|
Fosters a powerful collaboration between leading institutes in Victoria and a sharing of resources and expertise |
Yes |
Yes |
No |
|
Improve industrial production efficiency which has major environmental implications |
Yes |
Yes |
No |
|
Employ additional post-doctoral researchers and research assistants and to educate existing researchers in the use of cutting edge technologies |
Yes |
Yes |
Yes |
Source: Victorian Auditor-General's Office.
Many of the proposed benefits are reasonable and may be achievable, however, the business plan does not specify what measures should be adopted to assess the achievement of these benefits.
Actual benefits
Outcomes in relation to strategic goals such as skills base, commercial and collaboration, scientific research, attraction of overseas experts and investment were identified.
Some reported benefits were not measured or specified in any detail and some captured achievements should be more correctly described as outcomes or outputs rather than benefits.
Plans for benefits management
There was no evidence of the existence or use of documented benefit management plans or any other equivalent documents by DBI.
Post-implementation reviews
The final report submitted to DBI from the grant recipient and STI monitoring surveys identified key achievements of the project. However, many of the achievements should be regarded as outcomes or outputs.
Many expected benefits have not been tracked or measured by DBI.
Centre for Drug Candidate Optimisation
Conclusion
Achievements of expected benefits cannot be assessed accurately without describing how or when to measure the expected benefits, specifying targets, or by having a baseline against which progress can be measured.
Some benefits were identified from the investment; however, delivery of expected benefits was not specifically measured and reported.
Overview of the investment
The Centre for Drug Candidate Optimisation has been allocated $4 million in instalments from the first generation STI Initiative's contestable process since October 2002.
Over the past decade, a general increase in drug discovery and drug synthesis technologies, together with the decoding of the human genome, has led to a large number of potential drug candidates.
Drug candidate optimisation studies use chemical, biological and pharmaceutical research to evaluate potential candidates during the development phase of new drugs. This helps to identify the most appropriate properties from the vast numbers of potential drug candidates emerging from drug discovery studies.
The optimisation process can increase the probability of new drug candidates successfully progressing through human clinical studies, and thus making them more time and cost effective.
The centre aims to be the premier Australian facility for drug candidate optimisation by undertaking collaborative research to produce optimised drug candidates for commercial and non-commercial partners. It also develops new technology platforms to support its lead optimisation program.
Expected benefits
The project's business plan describes some private and public benefits that would be created through the project. However, many of the benefits are intangible in nature and difficult to measure.
Figure A9
Assessment of Centre for Drug Candidate Optimisation benefits against KPI criteria
|
Business plan |
Meaningful |
Attributable |
Measurable |
|---|---|---|---|
|
Developmentally-mature drug candidates with high commercial value and licensing appeal |
Yes |
Yes |
Yes |
|
A tangible increase in the market value of companies developing new drug candidates |
Yes |
Yes |
Yes |
|
Better protected intellectual property for the collaborating partners |
Yes |
Yes |
No |
|
A more conducive environment for early stage Victorian companies to attract private or venture equity |
Yes |
No |
No |
|
Enhanced appreciation of the commercial value of lead discoveries and the processes for enhancing that value |
Yes |
No |
No |
|
Ready access to a facility that integrates all the required experimental work to optimise pre-clinical drug candidates |
Yes |
Yes |
Yes |
|
Greater employment by establishing local pre-clinical capabilities |
Yes |
Yes |
Yes |
|
A facility that links to clinical development such as the CTV initiative |
No |
Yes |
Yes |
|
Greater motivation for investigators to pursue further development of their research |
Yes |
Yes |
No |
|
Supporting and developing linkages with important basic research groups |
No |
Yes |
Yes |
|
Facilitation of successful economic outcomes for discovery groups Victoria |
Yes |
Yes |
Yes |
|
Greater certainty of attracting venture capital and investment for drug discovery research and development |
Yes |
No |
No |
|
Positioning Victoria as the pre-eminent Australian region for new drug research |
Yes |
No |
No |
|
Acting as a catalyst for the maturation of pre-clinical development expertise in Australia |
Yes |
Yes |
No |
|
Supporting and crystallising high premium commercial returns on the recent investment made in biotechnology, pharmaceutical and veterinary research in Victoria |
Yes |
No |
No |
|
Demonstration to the basic science research community that high value-adding translational research can be effectively conducted within the University environment |
No |
Yes |
No |
|
Direct job creation (supporting a minimum of 19 jobs in the first three years) and skill base enhancement. |
Yes |
Yes |
Yes |
|
Training post-graduate students, broader education in pharmaceutical sciences, attracting the best students into higher degree research activities |
Yes |
Yes |
Yes |
|
Complementing new initiatives such as the Australian Synchrotron at Monash, and the proposed National Biotechnology Centre of Excellence |
Yes |
Yes |
No |
|
Linkage and integration with pre-clinical and clinical service providers |
No |
Yes |
Yes |
|
Public health benefits by supporting the development of therapeutics |
Yes |
No |
No |
Although there are numerous potential benefits identified, no specific measures were described to assess the delivery of these anticipated benefits.
Actual benefits
Outcomes in relation to skills base, commercial outcomes, scientific research, collaboration, and science awareness were identified in the STI outcome monitoring survey.
Social, economic and environmental benefits were identified. Although some benefits may have been captured, they were not measured or specified in any detail.
Plans for benefits management
There was no evidence of the existence or use of documented benefit management plans or any other equivalent documents by DBI.
Post-implementation reviews
The final project report submitted to DBI outlines some flow-on benefits of the investment. However, it does not describe when these benefits will be achieved, nor how they would be measured.
Many achievements captured in the STI monitoring survey and post-grant reports were outcomes or outputs rather than benefits.
Apart from the information captured on direct job creation, and training and education provided, other expected benefits have not been specifically measured. As a result, a conclusion cannot be formed on whether or not these expected benefits have been achieved.
Appendix B. Assessment of case study investments against criteria
This Appendix contains an assessment of the selected case studies against the evaluation criteria used for this audit's sub-objectives. The assessments use the objectives set by government or grant funding agreements and are not an assessment of the overall performance of the recipient entity.
The basis for the assessments contained in the table is as follows:
Most = Most of the attributes expected by the criteria.
Some = Some of the attributes expected by the criteria.
None = Does not display the attributes expected by the criteria.
Figure B1
Assessment of case study investments against audit criteria
| Australian Synchrotron | Bio21 Institute | Neurosciences Victoria | BioGrid | Victorian Tissue Bank Initiative | Australian Tissue Engineering Centre | Victorian Microarray Technology Consortium | Centre for Drug Candidate Optimisation | |
|---|---|---|---|---|---|---|---|---|
| Sub-objective 1: Is there clear alignment of programs and investments to the applicable biotechnology strategic development plans? | ||||||||
| Alignment, communication, coordination | ||||||||
|
Program and project objectives are reviewed to ensure they will make the necessary contribution to BSDPs and interface effectively with other government policy objectives and initiatives (a) |
Some | Some | Some | Some | Some | Some | Some | Some |
|
Projects have clear, realistic and unambiguous scope and specifications and have been considered in the wider context of interdependencies in the portfolio and other change programs |
Some | Some | Most | Most | Most | Most | Most | Most |
|
Business cases reflect an underlying investment logic, address specific needs, are robust, achievable, likely to deliver value-for-money, set out the deliverables and key results areas, and demonstrate the alternate options explored |
Some | Some | Most | Some | Most | Most | Most | Most |
|
Sub-objective 2: Are all program and investment outputs monitored, analysed and reported to assess the achievement of desired strategic outcomes? |
||||||||
|
Monitoring, analysis, reporting |
||||||||
|
Continued funding is linked to continued project justification, the meeting of performance targets and the likely achievement of outcomes |
Some | Some | Some | Some | Some | Some | Some | Some |
|
A process exists to update the business cases as required and analyse the impact of changes on initial investment logic and the strategic plan |
Some | Some | Most | Most | Most | Most | Most | Most |
|
Key performance indicators (KPIs) are meaningful, attributable, measurable, documented and used as the basis for monitoring and analysing the progress of programs, projects, outputs and outcomes |
Some | None | Some | None | Some | None | None | None |
|
Outline risk management plans have been developed to effectively manage emerging risks and maintain alignment with the strategic plan |
Some | Some | Some | Some | Some | Some | Some | Some |
|
Communication plans exist to capture and report stakeholder information |
Some | Some | Most | Some | Most | Most | Most | Most |
|
Sub-objective 3: Have benefits been realised? |
||||||||
|
Planning and lessons learned |
||||||||
|
Expected benefits are clearly articulated, realistic and measurable |
Some | Some | Some | Some | Some | Some | Some | Some |
|
Post-implementation reviews are conducted to assess if the business case was realistic and to what extent the expected benefits have been achieved |
Most | Some | Most | None | Some | Some | Some | Some |
|
Realisation, measurement, sustainability |
||||||||
|
Actual benefits are identified (expected, unintended, disbenefits) and sustainable |
Some | Some | Most | None | Some | Some | Some | Some |
|
Benefits management plans are documented and include KPIs which are used as the basis for monitoring, analysing and reporting progress (b) |
None | None | None | None | None | None | None | None |
Note: (a) If an investment pre-dates the release of a BSDP, then the BSDP was not relevant to the assessment.
Note: (b) If no measurements have been defined for KPIs then the case study will not rank better than red.
Source: Victorian Auditor-General's Office.
Appendix C. Audit Act 1994 section 16-submissions and comments
Introduction
In accordance with section 16(3) of the Audit Act 1994 a copy of this report was provided to the Australian Synchrotron Company Limited, Australian Synchrotron Holding Company Limited, Department of Business and Innovation, Melbourne Health and the University of Melbourne with a request for submissions or comments.
The submissions and comments provided are not subject to audit nor the evidentiary standards required to reach an audit conclusion. Responsibility for the accuracy, fairness and balance of those comments rests solely with the agency head.